An example of one reason I chose geophysics for my PhD rather than electronics is demonstrated in the following discussion. The work early in my career focused my attention towards what I call “data acquisition” – how do I acquire the information of interest? Not only was I interested in the “how” of taking measurements, I was interested in the “what” that I was measuring: I can acquire a temperature reading with a diode network – but what causes the temperature to change and how much will it change?
In this discussion, I talk of one … not-really-typical … means of measuring carbon dioxide. I’ve been involved with projects that study the atmosphere to one degree or another my entire career … from my “almost first” professional project as a bare-bones rookie to my “almost last” senior-senior position.
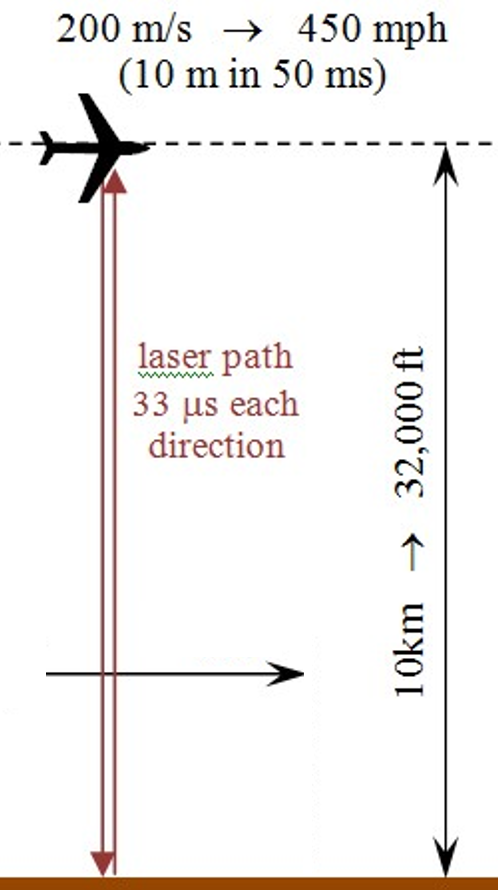
The goal is to measure atmospheric CO2 content over wide swaths of ground via airplane. The flight parameters called for two 4-hour flights per day; one 8-hr flight if feasible. The nominal aircraft speed was – and is – assumed to be about 450 mph … close enough to 200 m/s for a “best-case” analysis such as this.
(I dislike metric: based on prime numbers 2 and 5. The imperial system has better resolution (1°F vs 1°C?) and is based on prime numbers 2 and 3. I’ll mostly use metric herein. I use Windows for the same reason – ’cause that’s what most everyone expects.)
This works out to about 1800 miles/flight … about 2900 km. For estimation purposes, this allows 4 flight lines per sequence; each of roughly 400 miles (643.7376 km). Other than velocity, it is not necessary to consider this information at this time.
Flight altitude will be at/near the top of the troposphere* – about 33,000 ft (10.05843 km).
OK – call it 10.0 km.
*from NASA: “The troposphere is between 5 and 9 miles (8 and 14 kilometers) thick depending on where you are on Earth. It’s thinnest at the North and South Pole. This layer has the air we breathe and the clouds in the sky. The air is densest in this lowest layer. In fact, the troposphere contains three-quarters of the mass of the entire atmosphere.“
Airplane altitude is assumed constant although the specified altitude uncertainty in one potential platform is ![]() 5 m.
5 m.
The project is advertised to accurately measure atmospheric CO2 to 1 part per 400 … 0.25 %.
Could be a bit tight …
The dual-laser measurement system is based on differential molecular absorption.
Once I figure out what I’m measuring, I can begin to think of how to measure.
gdg
Table of Contents
Introduction
1: Abstract
2: Beer-Lambert and Optical Depth
3: Geometrical Construction – The Measurement Environment
4: Atmosphere Elements
5: Atmospheric Carbon Dioxide
6: Absorption Cross-Section
7: Plots
8: Basic Reflectivity & Range
9: Photodiodes and Signal Range
10: Avalanche Photodiodes
11: Ranging Radar
12: Step-Frequency Signal Structure
References
Basic Configuration
BBs As Photons; Photons As BBs
For this discussion, “light” can be considered as particles – photons. As such, one way to explain this method is to think of dropping BBs from the height through a cloud of magnets. Half the BBs are magnetic, the other half aren’t.
The non-magnetic BBs fall to a rough floor and bounce.Some bounce straight back up, the rest are scattered away.
The magnetic BBs fall with the non-magnetic ones. Some bounce straight back up, the rest are scattered away. But some are captured by the magnets.
Since the BBs fall together, and if there were no magnets, there should be close to the same number of magnetic and non-magnetic BBs that get returned. But there are magnets – if I count and compare the number of returned BBs of each type, I can estimate how many magnets there might be.
And The Light Fades
Leaving that analogy, a similar process is used to measure the decrease in light intensity when a beam of light travels through some “transparent” substance and eventually fades. Some photons are absorbed, others are scattered away – only a few are received.

This phenomena is described by the Beer-Lambert Law and the concept of “optical depth”.
So – off we go …
Next: 1 – Abstract
Up: Articles
